Technical Essay: Analysis of Selected Ambers from the Collections of the J. Paul Getty Museum
- Jeff Maish
- Herant Khanjian
- Michael R. Schilling
Introduction
Amber has been appreciated since antiquity for its unique aesthetic qualities in the production of small decorative objects. It has been a source of both mystery and curiosity, as it bridges the divide between the living and organic and the mineral and inorganic. It was initially selected for qualities such as color and hardness, with an eye toward an end market in jewelry production, and the Baltic Sea coastline has been, and continues to be, the largest source of the material.
The focus of amber studies over the past two hundred years has paralleled scientific developments in instrumentation and methods. Some of the earliest investigators used microscopy to view a hidden world of natural history and provide insights into past geological ages. More recent studies have analyzed the material itself in an attempt to better understand its chemistry, origins, and deterioration processes. This has included the identification of imitation ambers composed of natural and human-made compounds.1
Amber Characteristics
Although amber types have been classified generally, some ambiguities remain. Visual characteristics of amber such as color and translucency do not clearly relate to differences in chemical composition,2 and some differences may relate more closely to inclusions, entrapment of air, and states of oxidation. Amber may also be defined by grade, color, or even geographic origin, such as Romanian or Sicilian. Ambers such as Baltic may be further subdivided into the categories allingite, beckerite, gedanite, or glessite, based in part on opacity, color, and friability.3 Some subdivisions are also morphological. For example, amber with many tiny bubbles may be termed “bone” amber, whereas “foamy” amber has slightly larger bubbles. Amber typing can, therefore, be viewed from different perspectives ranging from morphological to chemical.
Amber Deterioration and Conservation
Although amber may have lain relatively dormant in geological deposits for thousands of years, its relatively recent collection, shaping, use, and reburial have often resulted in continued—and in some instances severe—deterioration. In general, deterioration manifests itself as a thick “corrosion” crust that not only obscures the translucent quality of amber but may also lead to flaking and loss of the carved surface. In the worst-case scenarios, the carved surface completely flakes off, leaving an ambiguously shaped amber core. Deterioration may continue in a collection’s environment and be aggravated by pollutants, oxidation processes, and inappropriate environmental controls.4 Recently, the degradation mechanisms and conservation treatments of archaeological amber have been studied using a variety of analytical instrumentation.5
Over the years, restorers and, more recently, conservators have attempted to reinforce fragile amber surfaces by applying a range of consolidative organic materials. Examples of past amber consolidants include dammar resin and “amber oil,” a product of amber distillation.6 A variety of waxes and natural and synthetic resins have also been applied. While preserving the morphological characteristics of carved amber, organic consolidants may interfere with future attempts to analyze or classify the amber. Therefore, the consolidation process should be carefully considered and, if carried out, fully documented.7
Scientific Analysis of Amber
The study of amber has kept pace over the past two centuries with the developments in scientific analysis. Microscopic studies beginning in the eighteenth century focused on the morphological characteristics of amber and the recognition of amber’s botanical origins.8 As methods for chemical analysis developed, so did the understanding of amber’s complex chemical structure.9 Considering the archaeological context of many amber finds, its characterization is further complicated by material degradation and possible interference from past stabilization treatments.10 Beginning in the 1960s, analytical studies of amber relied heavily on infrared spectroscopy (IR)11 and nuclear magnetic resonance (NMR).12
IR spectroscopy in particular was the first technique capable of readily identifying Baltic amber through the presence of a distinct succinic acid peak or “shoulder” in its infrared spectrum. However, the limits of this method were reached when it proved less successful in distinguishing among non-Baltic ambers. More-recent analytical studies have employed Raman spectroscopy,13 capillary gas chromatography / mass spectrometry (GC/MS),14 and pyrolysis–gas chromatography / mass spectrometry (Py-GC/MS),15 which are capable of isolating a broad range of compounds that compose amber.16 Combined with other analysis, this has led to proposals for the botanical origins of some ambers as well as common sourcing for previously distinct ambers.17
Current Research
The primary goal of the scientific investigation of a group of amber objects from the collection of the J. Paul Getty Museum was to verify that the ambers were indeed of Baltic origin. A secondary aim was to ascertain whether treatment with amber oil or other organic materials might interfere with the identification process. Samples were removed from the cores of twenty-six amber objects for analysis at the Getty Conservation Institute using Fourier-transform infrared spectroscopy (FTIR) and pyrolysis–gas chromatography / mass spectrometry with tetramethylammonium hydroxide for thermally-assisted hydrolysis and methylation (THM-Py-GC/MS). Surface samples were also removed from seven amber objects, in order to better understand the composition of weathered amber surfaces. For comparative purposes, tests were carried out on a number of reference materials, including Baltic amber, Dominican amber, copal resin, pine resin, sandarac resin, dried residue from amber-oil distillate, and amber varnish.
Fourier-Transform Infrared Spectrometry Procedure
The samples were analyzed on a Nic-plan infrared microscope equipped with a nitrogen-cooled MCT/A detector. Selected amber particles were placed on an infrared diamond window, flattened with a metal roller, and analyzed using a transmitted infrared beam apertured to 100 x 100 microns. The spectra are the sum of 100 scans at a resolution of 4 cm-1. Infrared analysis of the samples produced spectra containing bands that correspond to amber. For example, a characteristic peak attributed to the carbon-oxygen bond at 1158 cm-1 distinguishes Baltic amber. Additional bands at 1737 and 1715 cm-1are assigned to the ester and carboxylic acid groups, whereas peaks located at 1643 and 888 cm-1 are attributed to the exocyclic methylene group. Other components may be present in the samples at concentrations below the detection limit (5%).
THM–Pyrolysis–Gas Chromatography / Mass Spectrometry Procedure
Samples were tested on an HP 5972 gas chromatograph / mass spectrometer using a CDS Pyroprobe 2000, fitted with a valved interface at 330°C and purged with helium at 25 ml/minute. The split injector was at 340°C (30:1 ratio), and the MS transfer line was set to 300°C. A DB-5MS capillary column (30 M x 0.25 mm x 0.25 µm) was used, with helium at 44 cm/sec. The GC oven temperature program was 2 minutes at 40°C, then rising 6°C/minute to 310°C, and 13-minute isothermal. The solvent delay was 2.5 minutes. The mass spectrometer was scanned from m/z 35–700. Samples were placed into quartz tubes fitted with quartz wool, and three microliters of 25% tetramethyl ammonium hydroxide (TMAH) in methanol were introduced for derivatization. After 3 minutes, the tube was placed into a coiled filament probe, which was inserted into the valved interface. After purging for 3 seconds before pyrolysis, samples were pyrolyzed using the following temperature program: 200°C for 1 second, then ramped at 10°C/millisecond to 700°C, and held isothermally for 10 seconds.
Figure 1, an overlay of the FTIR spectra for Baltic amber and an amber object (, cat. no. 37), reveals characteristic spectral differences that make it possible to positively identify Baltic amber. The infrared spectrum of Baltic amber shows characteristic intense absorption bands at 2926, 2868, and 2849 cm-1, attributed to C-H stretching modes of the CH2 and CH3 groups. A doublet for carbonyl C=O stretching peaks at 1738 and 1715 cm-1 is characteristic of ester and acid groups. Additional bands at 1259 and 1158 cm-1 are assigned to CO-O- modes of the succinate group, whereas the C-H bending modes for the terminal olefins are located at 888 cm-1. Finally, the peak located at 1643 cm-1 is attributed to the exocyclic methylene group.
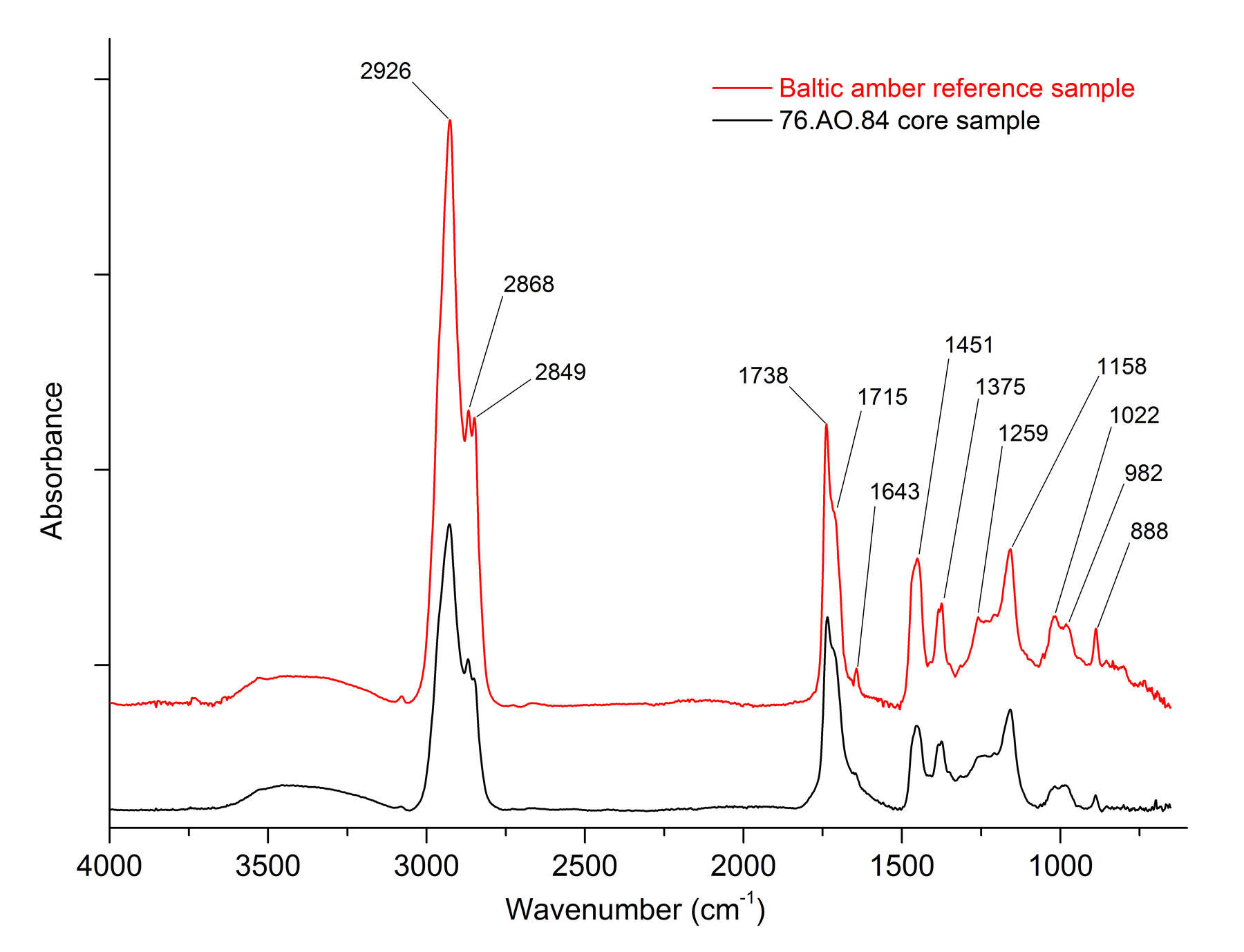 Figure 1
Figure 1In THM-Py-GC/MS results for Baltic and Dominican amber standards (figure 2), a total of 69 compounds were identified. Many of these are sesquiterpene and diterpene compounds that are abundant, though not especially characteristic of the type of amber, as well as numerous nonspecific compounds. Succinic acid is the dominant marker compound for Baltic amber, and it appears in the chromatogram as a large peak at 10.3 minutes. In this study, succinic acid was analyzed in the form of the dimethyl ester derivative, and is abbreviated in figures as succinate. In figure 3, which shows THM-Py-GC/MS results for amber object 82.A0.161.285, other Baltic amber marker compounds are present in varying amounts, including fenchol, borneol, camphene, and camphor. Two very small peaks identified as methyl fenchyl succinate and methyl bornyl succinate (Mills et al. 1984) may also appear in THM-Py-GC/MS results for Baltic ambers.
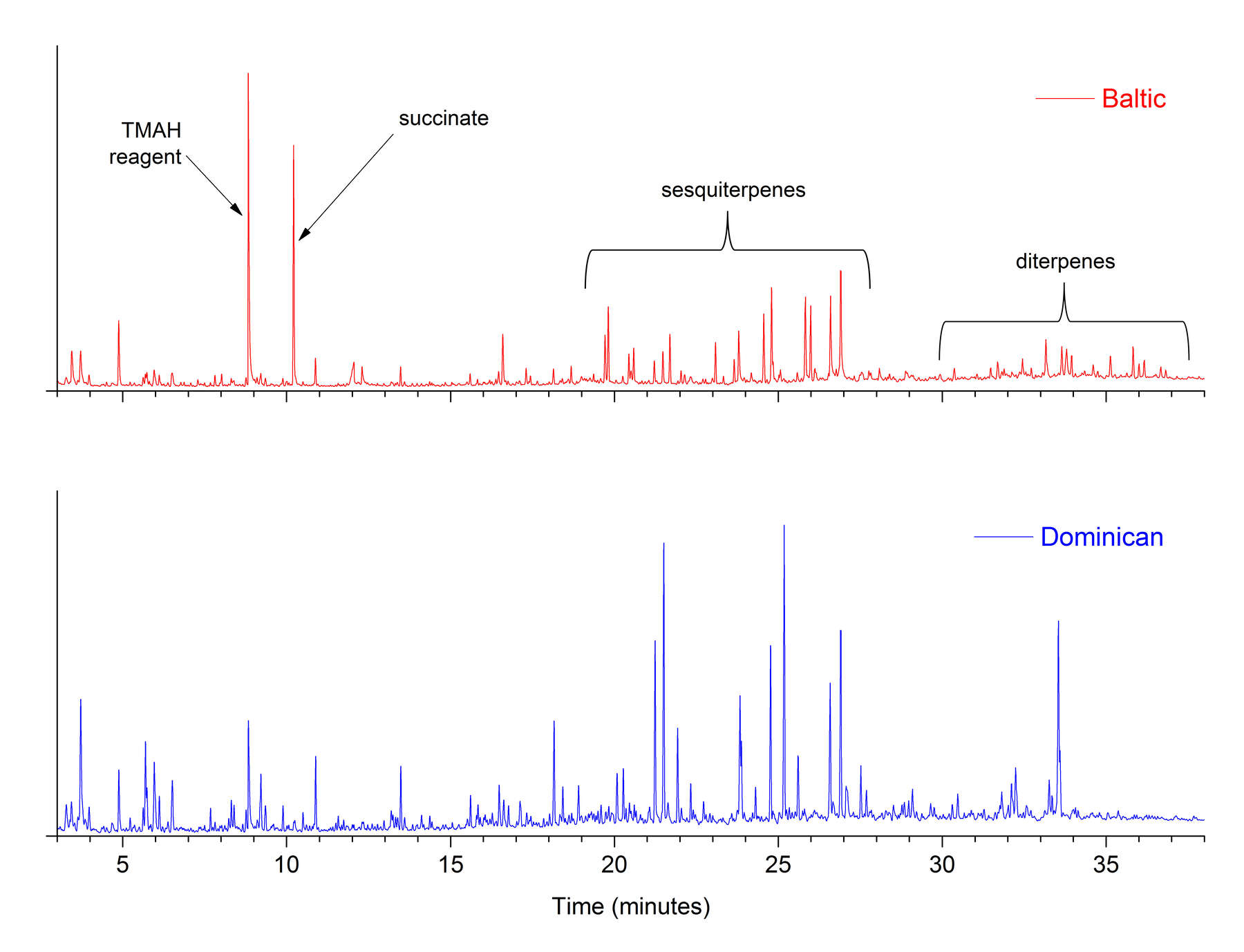 Figure 2
Figure 2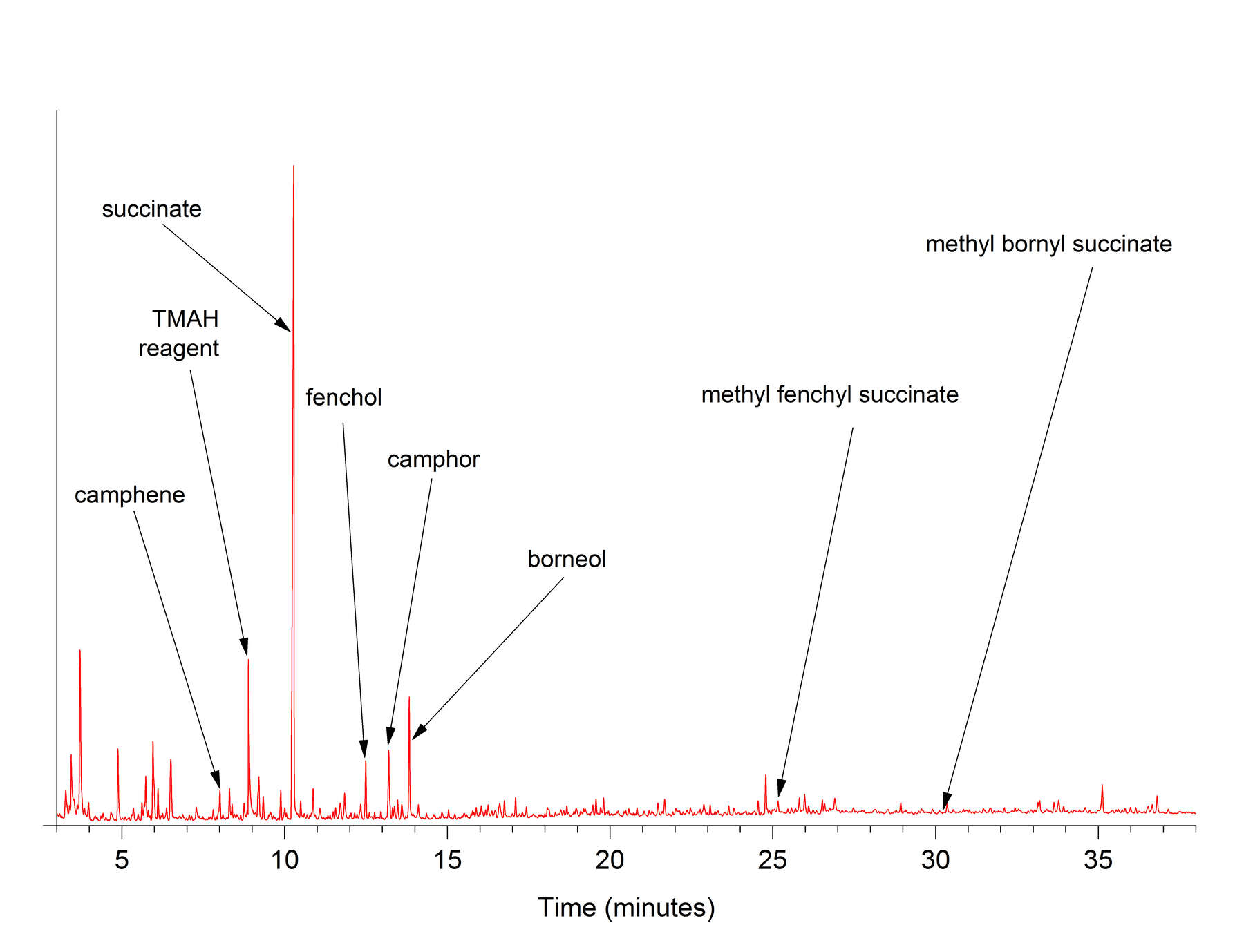 Figure 3
Figure 3Tables 1, 2, 3, and 4 list the various classes of compounds identified in the THM-Py-GC/MS analysis results of the amber objects and the reference materials. The identifications were based primarily on the results from mass spectral library searching using the NIST MS Search 2.0 program, and supplemented by published data (Mills et al. 1984). Although the NIST results of the nonspecific compounds listed in table 5 were inconclusive, the unknown compounds did appear on a rather consistent basis in the objects.
The THM-Py-GC/MS results for the reference samples appear in table 6. In this and all subsequent tables, the test results are expressed in terms of peak-area percentages relative to the total peak area for all of the compounds listed in tables 1, 2, 3, 4, and 5 (except for methyl fenchyl succinate, methyl bornyl succinate, and dibornyl succinate, which, due to their extremely small peak sizes, did not contribute significantly to the total peak area). Table 6 shows that the succinate content in the single known sample of Baltic amber was high, whereas almost no succinate was detected in the Dominican ambers, copal resin, sandarac resin, or pine resin. The succinate content in the ambers of unknown origin appeared rather variable, but the presence of the other markers in table 1 placed them firmly in the Baltic category. The “amber varnish” was found to contain a high concentration of a drying oil with no detectable succinate content. Fortunately, the test results for dried amber oil residue showed no significant amount of any of the Baltic marker compounds listed in table 1 except for borneol, indicating that amber oil treatment should not produce a “false positive” identification for Baltic amber.
In the THM-Py-GC/MS results for the core samples from the untreated amber objects (table 7), the most striking feature is the remarkably broad range for the succinate content compared to the composition of the standards. In an overlay of FTIR spectra for some of these samples (figure 4), the main trend is the shift of the carbonyl peak to a lower wavenumber with increasing succinate content, which is characteristic of the conversion of esters to carboxylic acids. These results provide evidence that partial hydrolysis of the succinate esters in the objects has occurred, which is a reaction that would enrich the residual amber in succinic acid.
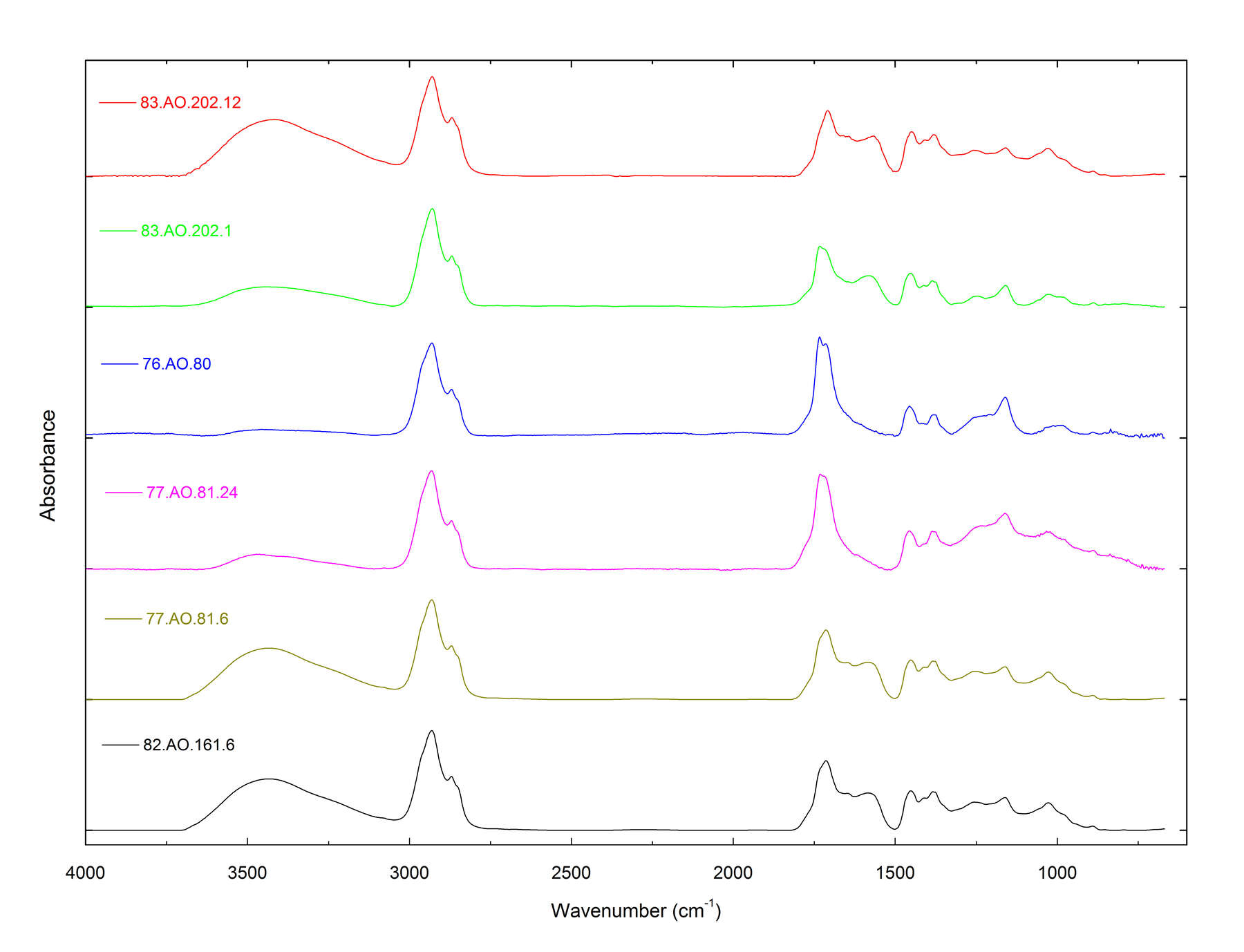 Figure 4
Figure 4One concern in this study was that the composition of the surface crusts of the amber objects might be considerably different from that of the inner cores, due to hydrolysis, weathering, handling, and treatment. This is why core samples were removed from the objects by microdrilling. In figure 5, the THM-Py-GC/MS results for the dark surface and inner core of a large piece of reference amber, it is clear that the surface has become partially depleted in succinate, with few other changes apparent. Table 8 shows the results for pairs of surface and core samples from the amber objects, and figure 6 shows a typical chromatographic result (for , cat. no. 12). The surfaces of these objects have also been depleted in succinate, but the sesquiterpenes and diterpenes also have been radically reduced. These compounds are not chemically bound to the polymeric network of the amber, which would make them more susceptible to leaching during burial.
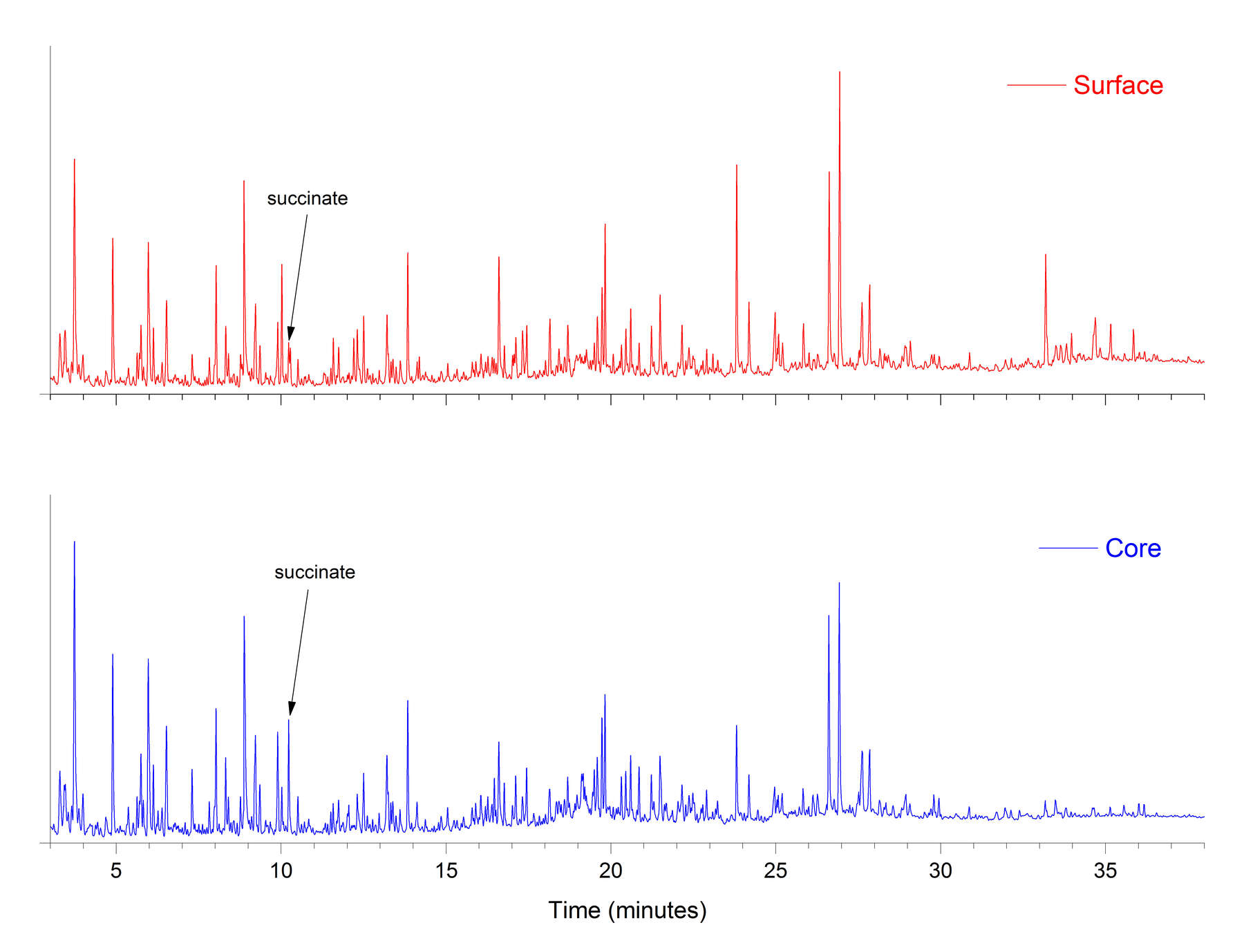 Figure 5
Figure 5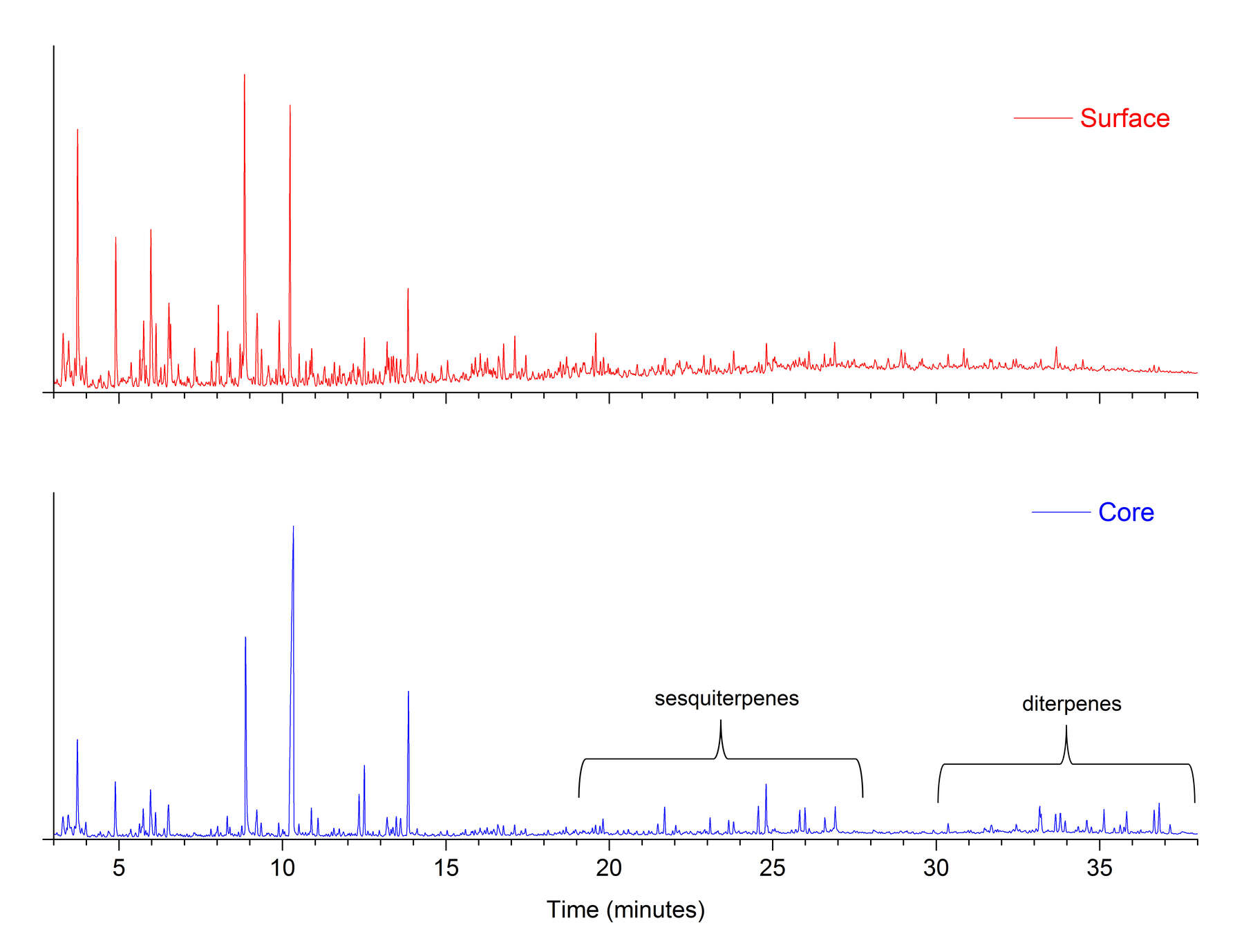 Figure 6
Figure 6FTIR analysis also reveals important details about the nature of the surface and core compositions. Figure 7 shows FTIR spectra for surface and core samples from . The saturated C-H bands at 2927 and 2869 cm-1 in the spectrum of the surface sample are reduced, whereas the C-O stretching modes at 1159 cm1 in the fingerprint region are more intense. This indicates that the surface is more highly oxidized than the core. The other important peak appears at 1574 cm-1, which is due to salts of succinic acid. There is a much higher concentration of succinate salts in the surface sample, which is consistent with exposure to alkaline conditions during some period of time.18 This might have occurred during burial, or resulted from harsh cleaning with alkaline chemicals. FTIR spectra of two surface samples and a core from (cat. no. 24) (figure 8) show an increased O-H stretching band in the surface sample, with a shift in the C=O band to lower wavenumbers, indicating the prevalence of carboxylic acids. However, the succinate salt peak at 1574 cm-1 is only a slight shoulder on the carbonyl peak, indicating that this object was not exposed to the same harsh alkaline conditions as .
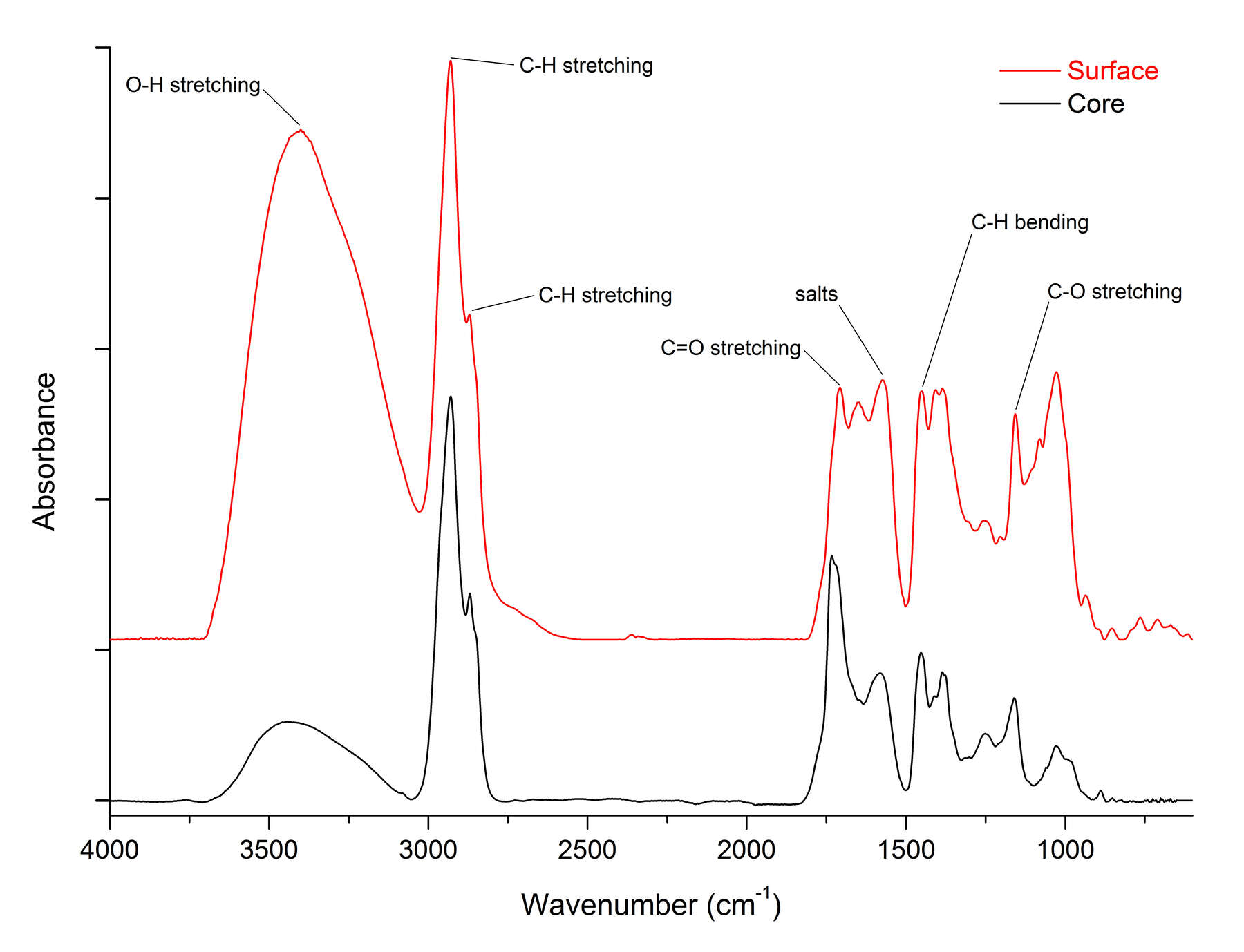 Figure 7
Figure 7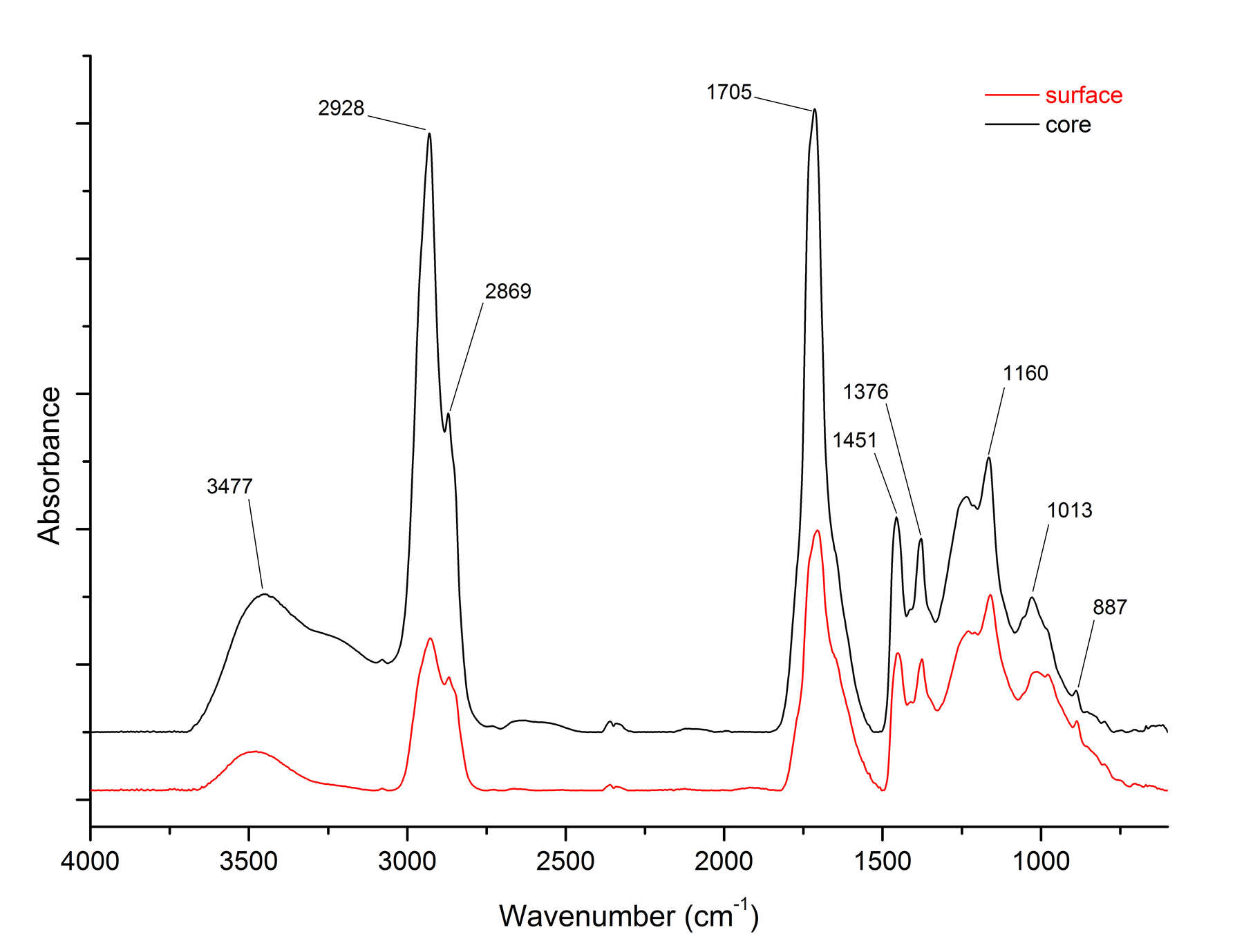 Figure 8
Figure 8Table 9 lists the THM-Py-GC/MS results for the treated amber objects, and representative chromatograms are shown in figure 9. Azelaic acid was detected in three of the objects: (cat. no. 16), (cat. no. 23), and (cat. no. 25). This is a common marker compound for cross-linked drying oils, and its presence along with palmitic acid and stearic acid indicates that drying oils may have been applied to these objects in an alternative type of conservation treatment. In (cat. no. 14), palmitic and stearic acids were detected along with cholesterol, but azelaic acid was absent. This suggests that an animal fat could have been applied to this object as another type of alternative treatment. Three amber objects tested in this study had been previously treated with amber oil: (cat. no. 1), (cat. no. 38), and (cat. no. 41). Their extremely high succinate contents suggest that they were highly degraded prior to treatment. In figure 10, the FTIR spectra for selected treated samples show that treatment with drying oil or amber oil does not interfere with the identification of Baltic amber.
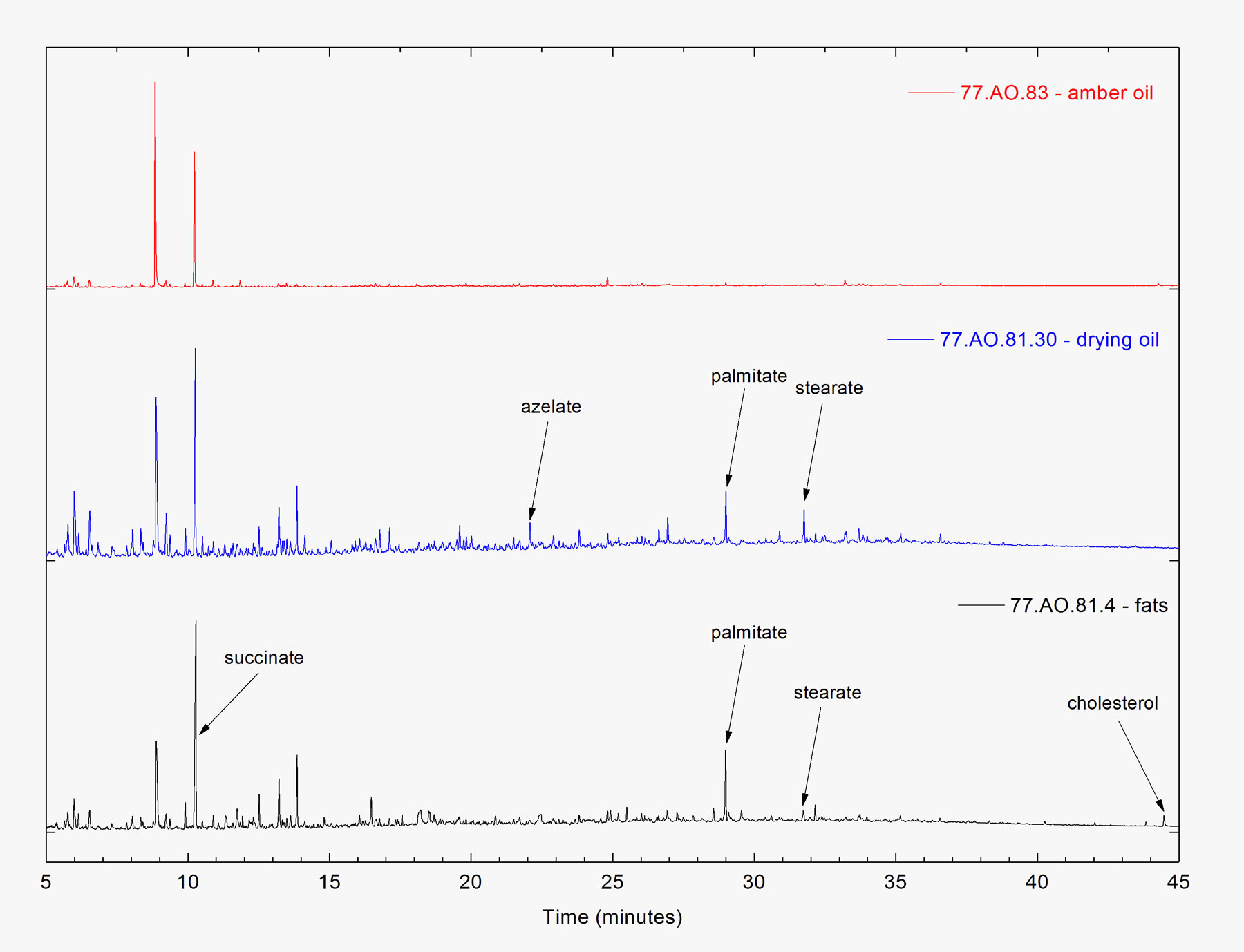 Figure 9
Figure 9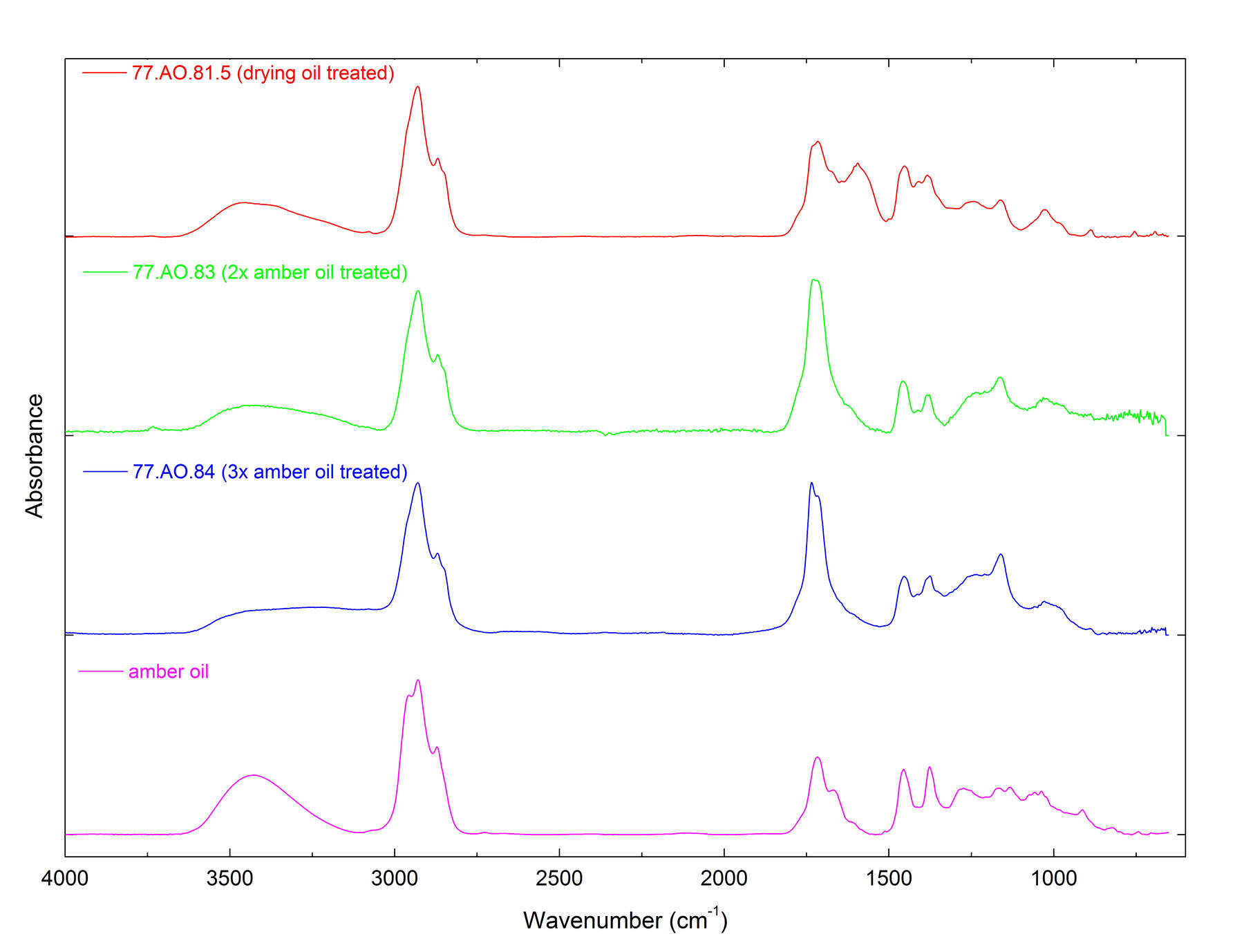 Figure 10
Figure 10Conclusions
This study has demonstrated that chemical analysis using FTIR and THM-Py-GC/MS can provide rich details concerning the composition of antique amber objects. Fundamentally, the analytical results showed that all of the amber objects in the Getty Museum are classified as Baltic amber. Additional information revealed the nature and extent of deterioration, and provided tantalizing hints about the nature of the burial conditions to which some of these objects may have been exposed. Finally, detection of certain marker compounds has shown that a number of amber objects were treated with drying oils and fats and, furthermore, that amber oil treatment does not interfere with the provenancing process.
Tables
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Bicyclo[2.2.1]heptane, 2,2-dimethyl-3-methylene- | Camphene | 79-92-5 | C10H16 | 136 | 8.04 |
| Butanedioic acid, dimethyl ester | Succinic acid, dimethyl ester | 106-65-0 | C6H10O4 | 146 | 10.27 |
| Bicyclo[2.2.1]heptane, 2-methoxy-1,3,3-trimethyl- | Methyl fenchyl ether | N/A | C11H20O | 168 | 12.35 |
| Bicyclo[2.2.1]heptan-2-ol, 1,3,3-trimethyl- | Fenchyl alcohol | 1632-73-1 | C10H18O | 154 | 12.50 |
| Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1S)- | L-camphor | 464-48-2 | C10H16O | 152 | 13.20 |
| Bicyclo[2.2.1]heptan-2-methoxy, 1,7,7-trimethyl-, (1S-endo)- | Methyl bornyl ether | N/A | C11H20O | 168 | 13.48 |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1S-endo)- | L-borneol | 464-45-9 | C10H18O | 154 | 13.83 |
| Naphthalene, 1,2,3,4-tetrahydro-1,8-dimethyl- | 25419-33-4 | C12H16 | 160 | 17.44 | |
| Cedren-13-methoxy, 8- | N/A | C16H26O | 234 | 24.80 | |
| Methyl fenchyl succinate | N/A | C15H24O4 | 268 | 25.16 | |
| Methyl bornyl succinate | N/A | C15H24O4 | 268 | 26.54 | |
| Cedren-13-ol, 8- | 18319-35-2 | C15H24O | 220 | 26.92 | |
| Dibornyl succinate | N/A | C24H38O4 | 390 | 38.64 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Bicyclo[2.2.1]heptane, 2,2-dimethyl-3-methylene- | Camphene | 79-92-5 | C10H16 | 136 | 8.04 |
| Butanedioic acid, dimethyl ester | Succinic acid, dimethyl ester | 106-65-0 | C6H10O4 | 146 | 10.27 |
| Bicyclo[2.2.1]heptane, 2-methoxy-1,3,3-trimethyl- | Methyl fenchyl ether | N/A | C11H20O | 168 | 12.35 |
| Bicyclo[2.2.1]heptan-2-ol, 1,3,3-trimethyl- | Fenchyl alcohol | 1632-73-1 | C10H18O | 154 | 12.50 |
| Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1S)- | L-camphor | 464-48-2 | C10H16O | 152 | 13.20 |
| Bicyclo[2.2.1]heptan-2-methoxy, 1,7,7-trimethyl-, (1S-endo)- | Methyl bornyl ether | N/A | C11H20O | 168 | 13.48 |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1S-endo)- | L-borneol | 464-45-9 | C10H18O | 154 | 13.83 |
| Naphthalene, 1,2,3,4-tetrahydro-1,8-dimethyl- | 25419-33-4 | C12H16 | 160 | 17.44 | |
| Cedren-13-methoxy, 8- | N/A | C16H26O | 234 | 24.80 | |
| Methyl fenchyl succinate | N/A | C15H24O4 | 268 | 25.16 | |
| Methyl bornyl succinate | N/A | C15H24O4 | 268 | 26.54 | |
| Cedren-13-ol, 8- | 18319-35-2 | C15H24O | 220 | 26.92 | |
| Dibornyl succinate | N/A | C24H38O4 | 390 | 38.64 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Podocarp-8-en-15-oic acid, 13alpha-methyl-13-vinyl-, methyl ester | Methyl pimara-8,15-dien-18-oate | 19907-21-2 | C21H32O2 | 316 | 33.17 |
| Podocarpa-8,11,13-trien-15-oic acid, 13-isopropyl-, methyl ester | Methyl dehydroabietate | 1235-74-1 | C21H30O2 | 314 | 35.14 |
| Methyl 5-(5,5,8a-trimethyl-2-methylenedecahydro-1-naphthalenyl)-3-methylpentanoate | Labd-8(20)-en-15-oic acid, methyl ester | 13008-80-5 | C21H36O2 | 320 | 33.49 |
| Methyl pimar-7-en-18-oate | 72088-13-2 | C21H34O2 | 318 | 33.80 | |
| Labda-8(20),12,14-trien-19-oic acid, methyl ester, (Z)- | Methyl cis-Communate | 10178-35-5 | C21H32O2 | 316 | 33.95 |
| Podocarp-8(14)-en-15-oic acid, 13á-methyl-13-vinyl-, methyl ester | Methyl sandaracopimarate | 1686-54-0 | C21H32O2 | 316 | 33.95 |
| Podocarp-7-en-15-oic acid, 13á-methyl-13-vinyl-, methyl ester | Methyl isopimarate | 1686-62-0 | C21H32O2 | 316 | 34.61 |
| Podocarpa-7,13-dien-15-oic acid, 13-isopropyl-, methyl ester | Methyl abietate | 127-25-3 | C21H32O2 | 316 | 35.84 |
| Podocarpa-6,8,11,13-tetraen-15-oic acid, 13-isopropyl-, methyl ester | Methyl 6-dehydrodehydroabietate | 18492-76-7 | C21H28O2 | 312 | 36.43 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Podocarp-8-en-15-oic acid, 13alpha-methyl-13-vinyl-, methyl ester | Methyl pimara-8,15-dien-18-oate | 19907-21-2 | C21H32O2 | 316 | 33.17 |
| Podocarpa-8,11,13-trien-15-oic acid, 13-isopropyl-, methyl ester | Methyl dehydroabietate | 1235-74-1 | C21H30O2 | 314 | 35.14 |
| Methyl 5-(5,5,8a-trimethyl-2-methylenedecahydro-1-naphthalenyl)-3-methylpentanoate | Labd-8(20)-en-15-oic acid, methyl ester | 13008-80-5 | C21H36O2 | 320 | 33.49 |
| Methyl pimar-7-en-18-oate | 72088-13-2 | C21H34O2 | 318 | 33.80 | |
| Labda-8(20),12,14-trien-19-oic acid, methyl ester, (Z)- | Methyl cis-Communate | 10178-35-5 | C21H32O2 | 316 | 33.95 |
| Podocarp-8(14)-en-15-oic acid, 13á-methyl-13-vinyl-, methyl ester | Methyl sandaracopimarate | 1686-54-0 | C21H32O2 | 316 | 33.95 |
| Podocarp-7-en-15-oic acid, 13á-methyl-13-vinyl-, methyl ester | Methyl isopimarate | 1686-62-0 | C21H32O2 | 316 | 34.61 |
| Podocarpa-7,13-dien-15-oic acid, 13-isopropyl-, methyl ester | Methyl abietate | 127-25-3 | C21H32O2 | 316 | 35.84 |
| Podocarpa-6,8,11,13-tetraen-15-oic acid, 13-isopropyl-, methyl ester | Methyl 6-dehydrodehydroabietate | 18492-76-7 | C21H28O2 | 312 | 36.43 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Hexanedioic acid, dimethyl ester | dimethyl adipate | 627-93-0 | C8H28O4 | 174 | 15.47 |
| Heptanedioic acid, dimethyl ester | dimethyl pimelate | 1732-08-7 | C9H16O4 | 188 | 17.78 |
| Octanedioic acid, dimethyl ester | dimethyl suberate | 1732-09-8 | C10H18O4 | 202 | 20.02 |
| Dodecanoic acid, methyl ester | methyl laurate | 111-82-0 | C13H26O2 | 214 | 21.63 |
| Nonanedioic acid, dimethyl ester | dimethyl azelate | 1732-10-1 | C11H20O4 | 216 | 22.10 |
| Decanedioic acid, dimethyl ester | dimethyl sebacate | 106-79-6 | C12H22O4 | 230 | 24.03 |
| Tetradecanoic acid, methyl ester | methyl myristate | 124-10-7 | C15H30O2 | 242 | 25.48 |
| Hexadecanoic acid, methyl ester | methyl palmitate | 112-39-0 | C17H34O2 | 270 | 28.96 |
| 9-Octadecenoic acid (Z)-, methyl ester | methyl oleate | 112-62-9 | C19H36O2 | 296 | 31.71 |
| Octadecanoic acid, methyl ester | methyl stearate | 112-61-8 | C19H38O2 | 298 | 32.13 |
| Eicosanoic acid, methyl ester | methyl arachidate | 1120-28-1 | C21H42O2 | 326 | 35.01 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Hexanedioic acid, dimethyl ester | dimethyl adipate | 627-93-0 | C8H28O4 | 174 | 15.47 |
| Heptanedioic acid, dimethyl ester | dimethyl pimelate | 1732-08-7 | C9H16O4 | 188 | 17.78 |
| Octanedioic acid, dimethyl ester | dimethyl suberate | 1732-09-8 | C10H18O4 | 202 | 20.02 |
| Dodecanoic acid, methyl ester | methyl laurate | 111-82-0 | C13H26O2 | 214 | 21.63 |
| Nonanedioic acid, dimethyl ester | dimethyl azelate | 1732-10-1 | C11H20O4 | 216 | 22.10 |
| Decanedioic acid, dimethyl ester | dimethyl sebacate | 106-79-6 | C12H22O4 | 230 | 24.03 |
| Tetradecanoic acid, methyl ester | methyl myristate | 124-10-7 | C15H30O2 | 242 | 25.48 |
| Hexadecanoic acid, methyl ester | methyl palmitate | 112-39-0 | C17H34O2 | 270 | 28.96 |
| 9-Octadecenoic acid (Z)-, methyl ester | methyl oleate | 112-62-9 | C19H36O2 | 296 | 31.71 |
| Octadecanoic acid, methyl ester | methyl stearate | 112-61-8 | C19H38O2 | 298 | 32.13 |
| Eicosanoic acid, methyl ester | methyl arachidate | 1120-28-1 | C21H42O2 | 326 | 35.01 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Methyl benzene | Toluene | 108-88-3 | C7H8 | 92 | 3.76 |
| 1,3-Dimethyl-1-cyclohexene | 2808-76-6 | C8H14 | 110 | 4.92 | |
| Benzene, 1,4-dimethyl- | p-Xylene | 106-42-3 | C8H10 | 106 | 5.99 |
| Benzene, 1,3-dimethyl- | m-Xylene | 108-38-3 | C8H10 | 106 | 5.99 |
| 2-Propenoic acid, 2-methyl-, methyl ester | Methyl methacrylate | 80-62-6 | C5H8O2 | 100 | 2.87 |
| Ethylbenzene | 100-41-4 | C8H10 | 106 | 5.77 | |
| Benzene, 1,2-dimethyl- | o-Xylene | 95-47-6 | C8H10 | 106 | 6.53 |
| Benzene, 1-ethyl-3-methyl- | Toluene, m-ethyl- | 620-14-4 | C9H12 | 120 | 8.33 |
| Benzene, 1-ethenyl-2-methyl- | o-Vinyltoluene | 611-15-4 | C9H10 | 118 | 9.24 |
| Benzenemethanol, 2,5-dimethyl- | 2,5-Dimethylbenzyl alcohol | 53957-33-8 | C9H12O | 136 | 9.36 |
| Benzene, 1,2,4-trimethyl- | Pseudocumene | 95-63-6 | C9H12 | 120 | 9.90 |
| 3,3,5,5-Tetramethylcyclopentene | 38667-10-6 | C9H16 | 124 | 10.89 | |
| Benzoic acid, methyl ester | Methyl benzoate | 93-58-3 | C8H8O2 | 136 | 11.85 |
| Naphthalene | 91-20-3 | C10H8 | 128 | 14.12 | |
| Naphthalene, 1-methyl- | 90-12-0 | C11H10 | 142 | 16.62 | |
| Naphthalene, 2-methyl- | 91-57-6 | C11H10 | 142 | 17.11 | |
| 1,2,3-Trimethylindene | 4773-83-5 | C12H14 | 158 | 18.68 | |
| Naphthalene, 1,6,7-trimethyl- | 2245-38-7 | C13H14 | 170 | 22.93 |
| IUPAC Name | Synonym | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|---|
| Methyl benzene | Toluene | 108-88-3 | C7H8 | 92 | 3.76 |
| 1,3-Dimethyl-1-cyclohexene | 2808-76-6 | C8H14 | 110 | 4.92 | |
| Benzene, 1,4-dimethyl- | p-Xylene | 106-42-3 | C8H10 | 106 | 5.99 |
| Benzene, 1,3-dimethyl- | m-Xylene | 108-38-3 | C8H10 | 106 | 5.99 |
| 2-Propenoic acid, 2-methyl-, methyl ester | Methyl methacrylate | 80-62-6 | C5H8O2 | 100 | 2.87 |
| Ethylbenzene | 100-41-4 | C8H10 | 106 | 5.77 | |
| Benzene, 1,2-dimethyl- | o-Xylene | 95-47-6 | C8H10 | 106 | 6.53 |
| Benzene, 1-ethyl-3-methyl- | Toluene, m-ethyl- | 620-14-4 | C9H12 | 120 | 8.33 |
| Benzene, 1-ethenyl-2-methyl- | o-Vinyltoluene | 611-15-4 | C9H10 | 118 | 9.24 |
| Benzenemethanol, 2,5-dimethyl- | 2,5-Dimethylbenzyl alcohol | 53957-33-8 | C9H12O | 136 | 9.36 |
| Benzene, 1,2,4-trimethyl- | Pseudocumene | 95-63-6 | C9H12 | 120 | 9.90 |
| 3,3,5,5-Tetramethylcyclopentene | 38667-10-6 | C9H16 | 124 | 10.89 | |
| Benzoic acid, methyl ester | Methyl benzoate | 93-58-3 | C8H8O2 | 136 | 11.85 |
| Naphthalene | 91-20-3 | C10H8 | 128 | 14.12 | |
| Naphthalene, 1-methyl- | 90-12-0 | C11H10 | 142 | 16.62 | |
| Naphthalene, 2-methyl- | 91-57-6 | C11H10 | 142 | 17.11 | |
| 1,2,3-Trimethylindene | 4773-83-5 | C12H14 | 158 | 18.68 | |
| Naphthalene, 1,6,7-trimethyl- | 2245-38-7 | C13H14 | 170 | 22.93 |
| IUPAC Name | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|
| Methyltricyclo[2.2.1.0(2,6)]heptane | 4601-85-8 | C8H12 | 108 | 6.15 |
| Cyclopentane, 2-ethylidene-1,1-dimethyl- | 56324-66-4 | C9H16 | 124 | 7.84 |
| 1,3,3-Trimethyl-2-(2-methyl-cyclopropyl)-cyclohexene | 285129-06-8 | C13H22 | 178 | 16.62 |
| 2-Buten-1-one, 1-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 35044-68-9 | C13H20O | 192 | 19.73 |
| 1,5,9,9-Tetramethyl-2-methylene-spiro[3.5]non-5-ene | N/A | C14H22 | 190 | 19.82 |
| Bicyclo[4.1.0]heptan-2-ol, 1beta-(3-methyl-1,3-butadienyl)-2alpha, 6beta-dimethyl-3beta-acetoxy- | N/A | C16H24O3 | 264 | 21.49 |
| 2-Methyl-4-(2,6,6-trimethylcyclohex-1-enyl)but-2-en-1-ol | 62924-17-8 | C14H24O | 208 | 21.70 |
| 8-Acetyl-5,5-dimethyl-nona-2,3,8-trienoic acid, methyl ester | 68799-74-6 | C14H20O3 | 236 | 23.09 |
| 2-Methyl-4-(2,6,6-trimethylcyclohex-1-enyl)but-2-en-1-ol | 62924-17-8 | C14H24O | 208 | 23.83 |
| 7a-Isopropenyl-4,5-dimethyloctahydroindene-4-carboxylic acid | N/A | C15H24O2 | 236 | 24.56 |
| 2-[5-(2,2-Dimethyl-6-methylene-cyclohexyl)-3-methyl-pent-2-enyl]-[1,4]benzoquinone | N/A | C21H28O2 | 312 | 25.17 |
| Acetic acid, (1,2,3,4,5,6,7,8-octahydro-3,8,8-trimethylnaphth-2-yl)methyl ester | 314773-27-8 | C16H26O2 | 250 | 25.83 |
| Acetic acid, 3-(6,6-dimethyl-2-methylenecyclohex-3-enylidene)-1-methylbutyl ester | N/A | C16H24O2 | 248 | 26.00 |
| IUPAC Name | CAS # | Formula | MW | Retention Time (min) |
|---|---|---|---|---|
| Methyltricyclo[2.2.1.0(2,6)]heptane | 4601-85-8 | C8H12 | 108 | 6.15 |
| Cyclopentane, 2-ethylidene-1,1-dimethyl- | 56324-66-4 | C9H16 | 124 | 7.84 |
| 1,3,3-Trimethyl-2-(2-methyl-cyclopropyl)-cyclohexene | 285129-06-8 | C13H22 | 178 | 16.62 |
| 2-Buten-1-one, 1-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 35044-68-9 | C13H20O | 192 | 19.73 |
| 1,5,9,9-Tetramethyl-2-methylene-spiro[3.5]non-5-ene | N/A | C14H22 | 190 | 19.82 |
| Bicyclo[4.1.0]heptan-2-ol, 1beta-(3-methyl-1,3-butadienyl)-2alpha, 6beta-dimethyl-3beta-acetoxy- | N/A | C16H24O3 | 264 | 21.49 |
| 2-Methyl-4-(2,6,6-trimethylcyclohex-1-enyl)but-2-en-1-ol | 62924-17-8 | C14H24O | 208 | 21.70 |
| 8-Acetyl-5,5-dimethyl-nona-2,3,8-trienoic acid, methyl ester | 68799-74-6 | C14H20O3 | 236 | 23.09 |
| 2-Methyl-4-(2,6,6-trimethylcyclohex-1-enyl)but-2-en-1-ol | 62924-17-8 | C14H24O | 208 | 23.83 |
| 7a-Isopropenyl-4,5-dimethyloctahydroindene-4-carboxylic acid | N/A | C15H24O2 | 236 | 24.56 |
| 2-[5-(2,2-Dimethyl-6-methylene-cyclohexyl)-3-methyl-pent-2-enyl]-[1,4]benzoquinone | N/A | C21H28O2 | 312 | 25.17 |
| Acetic acid, (1,2,3,4,5,6,7,8-octahydro-3,8,8-trimethylnaphth-2-yl)methyl ester | 314773-27-8 | C16H26O2 | 250 | 25.83 |
| Acetic acid, 3-(6,6-dimethyl-2-methylenecyclohex-3-enylidene)-1-methylbutyl ester | N/A | C16H24O2 | 248 | 26.00 |
| Sample | Supplier | GCI Identifier | Peak Area Percentages | ||
|---|---|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | |||
| Amber varnish | Zecchi | VARN0084 | 0 | 0 | 77 |
| Copal resin | JPGM | 0 | 36 | 0 | |
| Pine resin | GCI | NRES0244 | 0 | 74 | 0 |
| Sandarac resin | Verfmolen ‘De Kat’ | NRES0295 | 0 | 19 | 0 |
| Amber oil | JPGM | 1 | 1 | 3 | |
| Dominican amber | JPGM | 0 | 14 | 1 | |
| Amber (Dominican?) | JPGM | NRES0095 | 2 | 1 | 0 |
| Amber | Kremer | NRES0005 | 3 | 2 | 1 |
| Yellow amber | Verfmolen ‘De Kat’ | NRES0296 | 3 | 2 | 1 |
| Amber | Zecchi | 10 | 2 | 2 | |
| Baltic amber | JPGM | 12 | 12 | 0.4 | |
| Amber | Zecchi | NRES0305 | 14 | 4 | 1 |
| Amber | Kremer | NRES0004 | 20 | 6 | 1 |
| Amber | Kremer | NRES0171 | 20 | 4 | 1 |
| Sample | Supplier | GCI Identifier | Peak Area Percentages | ||
|---|---|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | |||
| Amber varnish | Zecchi | VARN0084 | 0 | 0 | 77 |
| Copal resin | JPGM | 0 | 36 | 0 | |
| Pine resin | GCI | NRES0244 | 0 | 74 | 0 |
| Sandarac resin | Verfmolen ‘De Kat’ | NRES0295 | 0 | 19 | 0 |
| Amber oil | JPGM | 1 | 1 | 3 | |
| Dominican amber | JPGM | 0 | 14 | 1 | |
| Amber (Dominican?) | JPGM | NRES0095 | 2 | 1 | 0 |
| Amber | Kremer | NRES0005 | 3 | 2 | 1 |
| Yellow amber | Verfmolen ‘De Kat’ | NRES0296 | 3 | 2 | 1 |
| Amber | Zecchi | 10 | 2 | 2 | |
| Baltic amber | JPGM | 12 | 12 | 0.4 | |
| Amber | Zecchi | NRES0305 | 14 | 4 | 1 |
| Amber | Kremer | NRES0004 | 20 | 6 | 1 |
| Amber | Kremer | NRES0171 | 20 | 4 | 1 |
| Accession # | Peak Area Percentages | ||
|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | |
| (cat. no. 21) | 13 | 1 | 0 |
| 20 | 10 | 0 | |
| 24 | 5 | 0.4 | |
| (cat. no. 18) | 25 | 12 | 0.4 |
| (cat. no. 11) | 27 | 11 | 0 |
| 28 | 12 | 0.6 | |
| (cat. no. 22) | 34 | 7 | 0.7 |
| 34 | 9 | 0.0 | |
| 82.AC.161.285 | 35 | 4 | 0.7 |
| (cat. no. 33) | 36 | 5 | 0.6 |
| (cat. no. 56) | 38 | 22 | 0.5 |
| (cat. no. 28) | 38 | 8 | 0.3 |
| (cat. no. 53) | 39 | 9 | 1.1 |
| (cat. no. 29) | 39 | 16 | 0 |
| (cat. no. 20) | 41 | 8 | 0.9 |
| (cat. no. 17) | 41 | 9 | 0.6 |
| (cat. no. 13) | 46 | 7 | 0.3 |
| (cat. no. 55) | 47 | 16 | 0.4 |
| (cat. no. 27) | 53 | 4 | 0.3 |
| (cat. no. 9) | 65 | 6 | 0.4 |
| average | 36 | 9.1 | 0.4 |
| standard deviation | 12 | 5.0 | 0.3 |
| Accession # | Peak Area Percentages | ||
|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | |
| (cat. no. 21) | 13 | 1 | 0 |
| 20 | 10 | 0 | |
| 24 | 5 | 0.4 | |
| (cat. no. 18) | 25 | 12 | 0.4 |
| (cat. no. 11) | 27 | 11 | 0 |
| 28 | 12 | 0.6 | |
| (cat. no. 22) | 34 | 7 | 0.7 |
| 34 | 9 | 0.0 | |
| 82.AC.161.285 | 35 | 4 | 0.7 |
| (cat. no. 33) | 36 | 5 | 0.6 |
| (cat. no. 56) | 38 | 22 | 0.5 |
| (cat. no. 28) | 38 | 8 | 0.3 |
| (cat. no. 53) | 39 | 9 | 1.1 |
| (cat. no. 29) | 39 | 16 | 0 |
| (cat. no. 20) | 41 | 8 | 0.9 |
| (cat. no. 17) | 41 | 9 | 0.6 |
| (cat. no. 13) | 46 | 7 | 0.3 |
| (cat. no. 55) | 47 | 16 | 0.4 |
| (cat. no. 27) | 53 | 4 | 0.3 |
| (cat. no. 9) | 65 | 6 | 0.4 |
| average | 36 | 9.1 | 0.4 |
| standard deviation | 12 | 5.0 | 0.3 |
| Accession # | Sample Location | Peak Area Percentages | ||
|---|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | ||
| core | 20 | 9.6 | 0.0 | |
| surface | 4.2 | 9.1 | 0.8 | |
| core | 25 | 12 | 0.4 | |
| surface | 16 | 4.6 | 1.6 | |
| (cat. no. 19) | core | 27 | 11 | 0.0 |
| surface | 11 | 1.7 | 1.5 | |
| core | 34 | 9.1 | 0.0 | |
| surface | 11 | 2.0 | 1.5 | |
| core | 38 | 8.1 | 0.3 | |
| surface | 29 | 4.3 | 0.7 | |
| core | 41 | 8.0 | 0.9 | |
| surface | 12 | 2.0 | 1.2 | |
| Accession # | Sample Location | Peak Area Percentages | ||
|---|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | ||
| core | 20 | 9.6 | 0.0 | |
| surface | 4.2 | 9.1 | 0.8 | |
| core | 25 | 12 | 0.4 | |
| surface | 16 | 4.6 | 1.6 | |
| (cat. no. 19) | core | 27 | 11 | 0.0 |
| surface | 11 | 1.7 | 1.5 | |
| core | 34 | 9.1 | 0.0 | |
| surface | 11 | 2.0 | 1.5 | |
| core | 38 | 8.1 | 0.3 | |
| surface | 29 | 4.3 | 0.7 | |
| core | 41 | 8.0 | 0.9 | |
| surface | 12 | 2.0 | 1.2 | |
| Accession # | Sample Location | Treatment | Peak Area Percentages | ||
|---|---|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | |||
| core | drying oil | 34 | 5.2 | 18 | |
| surface | drying oil | 13 | 4.6 | 7.7 | |
| core | drying oil | 29 | 6.4 | 6.6 | |
| core | fatty substance | 39 | 6.2 | 0.7 | |
| surface | 23 | 2.7 | 8.1 | ||
| core | amber oil, once | 72 | 5.1 | 0.6 | |
| surface | 44 | 7.6 | 0.4 | ||
| core | amber oil, twice | 38 | 5.6 | 1.9 | |
| core | amber oil, three times | 54 | 2.9 | 0.4 | |
| Accession # | Sample Location | Treatment | Peak Area Percentages | ||
|---|---|---|---|---|---|
| Succinate | Diterpenes | Fatty Acids | |||
| core | drying oil | 34 | 5.2 | 18 | |
| surface | drying oil | 13 | 4.6 | 7.7 | |
| core | drying oil | 29 | 6.4 | 6.6 | |
| core | fatty substance | 39 | 6.2 | 0.7 | |
| surface | 23 | 2.7 | 8.1 | ||
| core | amber oil, once | 72 | 5.1 | 0.6 | |
| surface | 44 | 7.6 | 0.4 | ||
| core | amber oil, twice | 38 | 5.6 | 1.9 | |
| core | amber oil, three times | 54 | 2.9 | 0.4 | |
Notes
- See N. Kalsbeek and K. Botfeldt, “Identification of Amber and Amber Imitations by Infrared Spectroscopy,” Meddelelser om konservering no. 1 (2007): 3–11. Imitations have included materials such as Bakelite, nitrocellulose, polystyrene, and plant resins. ↩
- See for a discussion of amber and its terminology. ↩
- E. Stout, C. Beck, and B. Kosmowska-Ceranowicz, for example, used infrared spectroscopy (IR) to compare and separate gedano-succinite from succinite: see “Gedanite and Gedano-Succinite,” in , pp. 130–48. ↩
- See J. Waddington and J. Fenn, “Preventive Conservation of Amber: Some Preliminary Investigations,” Collection Forum 4, no. 2, (Fall 1988): 25–31; and Y. Shashoua, National Museum of Denmark, 2002, http://www.natmus.dk/cons/reports/2002/amber/amber.pdf. ↩
- G. Pastorelli, “Archaeological Baltic Amber: Degradation Mechanisms and Conservation Measures” (Ph.D. diss., University of Bologna, 2009). ↩
- F. Preusser, “Zur Restaurierung von stark korrodiertem Bernstein” (“The Restoration of Badly Weathered Amber”), Arbeitsblätter für Restauratoren 9, no. 2 (1976): 75–77. ↩
- See N. Bromelle, C. Beck, and G. Thomson, “Authentication and Conservation of Amber: Conflict of Interests,” in Science and Technology in the Service of Conservation: Preprints of the Contributions to the Washington Congress, 3–9 September 1982, ed. N. Bromelle and G. Thomson (London, 1982), pp. 104–7. ↩
- The relationship of Baltic amber and succinic acid was recognized in the early nineteenth century by chemists in Germany, where succinic acid was isolated using strong acids and bases. One study identified the amber constituent camphor (borneol) through smell. ↩
- For an excellent overview of amber and resin studies see I. Angelini in G. Artioli, Scientific Methods and Cultural Heritage: An Introduction to the Application of Materials Science to Archaeometry and Conservation Science (New York, 2010). ↩
- Brommelle et al. 1982 (in n. 7, above) first warned that most conservation materials will interfere with infrared spectra. D. Thickett discusses the problems of consolidant removal and the effects of solvents on amber in “The Influence of Solvents on the Analysis of Amber,” in Conservation Science in the UK: Preprints of the Meeting Held in Glasgow, May 1993, ed. N. J. Tennent (London, 1993), pp. 49–56. ↩
- The earliest IR studies of amber were carried out most notably by J. Langenheim, C. Beck, and R. Rottländer. See, for example, C. Beck and H. Hartnett, “Sicilian Amber,” in , pp. 36–47; and C. Beck, “Spectroscopic Investigations of Amber,” Applied Spectroscopy Reviews 22, no. 1 (1986): 57–110. Amber studies have benefited from further developments such as Fourier-transform infrared spectroscopy (FTIR), diffuse-reflectance infrared Fourier-transform (DRIFT), and Attenuated Total Reflection (ATR) FTIR. For the DRIFT method, see I. Angelini and P. Bellintani, “Archaeological Ambers from Northern Italy: An FTIR-DRIFT Study of Provenance by Comparison with the Geological Amber Database,” Archaeometry 47, no. 2 (2005): 441–54. For the ATR method, see M. Guiliano, L. Asia, G. Onoratini, and G. Mille, “Applications of Diamond Crystal ATR FTIR Spectroscopy to the Characterization of Ambers,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 67, no. 5 (2007): 1407–11. ↩
- For a summary of the use of the nuclear magnetic resonance method in amber analysis, see Artioli, Scientific Methods and Cultural Heritage, p. 383 (in n. 9, above). ↩
- See Y. Shashoua et al., “Raman and ATR-FTIR Spectroscopies Applied to the Conservation of Archaeological Baltic Amber,” Journal of Raman Spectroscopy 37, no. 10 (September 2006). ↩
- J. Mills, R. White, and L. Gough, “The Chemical Composition of Baltic Amber,” Chemical Geology 47 (1984): 15–39. ↩
- See A. Shedrinsky et al., “The Use of Pyrolysis Gas Chromatography (PyGC) in the Identification of Oils and Resins Found in Art and Archaeology,” Conservation of Cultural Property in India 21 (1988): 35–41; and J. Boon, A. Tom, and J. Purveen, “Microgram Scale Pyrolysis Mass Spectrometric and Pyrolysis Gas Chromatographic Characterization of Geological and Archaeological Amber and Resin Samples,” in , pp. 9–27. For comprehensive overviews of the method, see “The Application of Analytical Pyrolysis to the Study of Cultural Materials,” chap. 6 in Applied Pyrolysis Handbook, 2nd ed., ed. Thomas Wampler (Boca Raton, FL, 2007), pp. 105–31. ↩
- Gas chromatography effectively separates volatile, solvent-extractable components, which are subsequently detected and analyzed by the mass spectrometer. The limit to analyzing only the extractable components is mostly overcome by pyrolyzing the sample before the analysis. Pyrolysis uses thermal energy to break down polymeric and nonvolatile materials into small volatile molecules that are amenable to gas chromatographic analysis. ↩
- E. Stout, C. W. Beck, and K. B. Anderson, “Identification of Rumanite (Romanian Amber) as Thermally Succinite (Baltic Amber),” Physics and Chemistry of Minerals 27, no. 9 (2000): 665–78. ↩
- Pastorelli 2009 (in n. 5, above). ↩
Bibliography
- Anderson and Crelling 1995
- Anderson, K. B., and J. C. Crelling, eds. Amber, Resinite, and Fossil Resins. Washington, DC, 1995.
- Beck and Bouzek 1993
- Beck, C. W., and J. Bouzek, eds. Amber in Archaeology: Proceedings of the Second International Conference on Amber in Archaeology, Liblice, 1990. Prague, 1993.
- Rice 2006
- Rice, P. C. Amber, the Golden Gem of the Ages. Bloomington, IN, 2006.